Kilocalorie per mole
The kilocalorie per mole is a derived unit of energy per Avogadro’s number of particles. It is the quotient of a kilocalorie (1000 thermochemical gram calories) and a mole, mainly used in the United States.
In SI units, it is equal to 4.184 kJ/mol, or 6.9477×10−21 J per molecule. At room temperature (25 °C, 77 °F, or 298.15 K) it is equal to 1.688 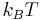 .
.
Physical quantities measured in kcal·mol−1 are usually thermodynamical quantities; mostly free energies such as: